Catalog
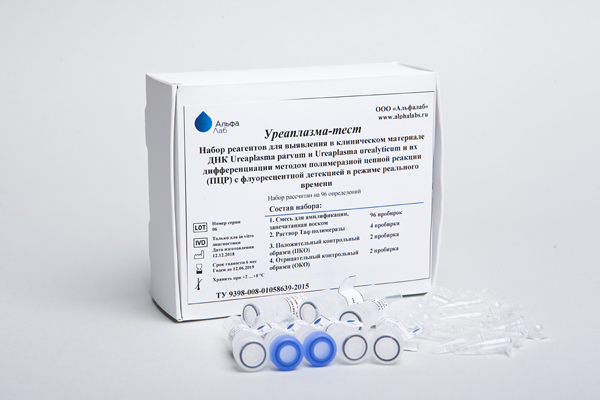
Ureaplasma test
The "Ureaplasma-test" reagent kit is intended for the qualitative detection of Ureaplasma parvum and Ureaplasma urealyticum DNA and their differentiation by the method of polymerase chain reaction (PCR) with fluorescence detection in real-time.
A study on the presence of DNA Ureaplasma parvum and Ureaplasma urealyticum is conducted to identify the causative agent of ureaplasma infection. The results of PCR studies are taken into account in the comprehensive diagnosis of inflammatory diseases of the urogenital tract. Determining the species of Ureaplasma is possible only on the basis of molecular genetic methods, in particular, the PCR method, and is necessary for choosing the optimal therapeutic strategy.
The material for the study are smears from the human urogenital tract.
The scope of the set is clinical laboratory diagnostics, scientific research. In vitro research only.